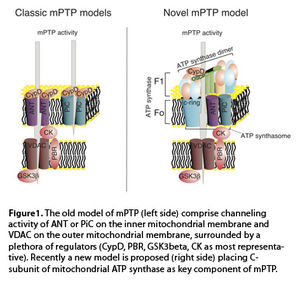
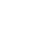The mitochondrial permeability transition (MPT) refers to an alteration of the permeability of the inner mitochondrial membrane and was characterized for the first time in 1979 (1). Interest regarding this peculiar mitochondrial state increased exponentially in the 1990s after the MPT was shown to be a strategic regulator of cell death (2). Although it was initially linked to reperfusion injury, it has now been established that the MPT can participate in both apoptosis (via the release of mitochondrial proapoptotic factors) and necrotic cell death (via impairment of ATP synthesis and deregulation of intracellular ionic gradients).
Since the proposal that a high conductivity channel may be responsible for the permeability transition state (3), the existence of a permeability transition pore (mPTP) has been hypothesized. Several studies have attempted to identify the molecular structure of this pore, revealing a complex organization with elements located in all the mitochondrial subcompartments. The initial model of mPTP proposes that the voltage dependent anion channel (VDAC) and the adenosine nucleotide transporter (ANT) are located on the outer mitochondrial membrane and inner mitochondrial membrane, respectively, as core components of the pore. These proteins are surrounded by a series of regulators, ranging from kinases (such as hexokinase II or glycogen synthase kinase 3 beta), the peripheral benzodiazepine receptor (PBR), members of the Bcl-2 family (with both positive and negative regulatory activity), and the peptidylprolyl isomerase F CypD (Figure 1) (4). Despite this proposed model, knockouts of both VDAC and ANT in animal models excluded these elements as pore forming components, placing them in the broad group of activity regulators. Some authors have suggested that the inorganic phosphate carrier (PiC) may be a possible structural element, if not the specific pore-forming element, of the mPTP (5).

Nonetheless, despite the extensive research performed in this field, a complete and clear model for mPTP activity is currently unavailable.
A series of studies have suggested that mitochondrial ATP synthase could be an important element of the mPTP. Evidence for this possibility includes the interaction with the regulators CypD and Bcl-Xl as well as the shared sensitivity to ions, including Mg2+ and Ca2+.
We recently identified the C subunit of mitochondrial ATPase as a fundamental regulator of mPTP activity (6). In fact, silencing C subunit expression can completely block MPT induction by Ca2+ and oxidants, whereas C subunit overexpression dramatically promotes MPT induction. C subunits are organized as oligomers in the inner mitochondrial membrane, known as the C-ring, and form part of the FO portion of ATP synthase. The rotation of this ring, driven by proton transport, is responsible for the conformational rearrangements that allow ATP synthesis. Isolated C-rings have been previously reported to possess gating capacity, which indicates that the C-ring itself could be the pore-forming region of mPTP. Nonetheless, the possibility that C-rings could exist outside ATP synthase in vivo has yet to be validated. Interestingly, a few months after our publication, it was proposed that only ATP synthase dimers are able to exert mPTP activity (7). This further raises more questions because ATP synthase dimers are usually associated with an increase in ATP production, which is inconsistent with mPTP activity.
Overall, further studies will be required to achieve a complete understanding of mPTP structure and activity. Nonetheless, these new concepts are intriguing, especially given the fact that ANT and PiC are known members of the so-called ATP synthasome, suggesting that the mPTP might be located within such a complex. These findings also imply a molecular identity for the link between cell death and cell survival, which is known to occur within mitochondria.
- Haworth, R. A. & Hunter, D. R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Archives of biochemistry and biophysics 195, 460–7 (1979).
- Crompton, M. & Costi, A. A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. The Biochemical journal 266, 33–9 (1990).
- Szabó, I. & Zoratti, M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. The Journal of biological chemistry 266, 3376–9 (1991).
- Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87, 99–163 (2007).
- Varanyuwatana, P. & Halestrap, A. P. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 12, 120–5 (2012).
- Bonora, M. et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell cycle (Georgetown, Tex.) 12, 674–83 (2013).
- Giorgio, V. et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proceedings of the National Academy of Sciences of the United States of America 110, 5887–92 (2013).