Cystic fibrosis (CF) is a common (one affected newborn out of 2500), autosomic recessive, genetic disease in the white population and is due to mutations in the ABCC7 gene. This gene codes for the cystic fibrosis transmembrane regulator (CFTR), an anion channel implicated in the transport of chloride and bicarbonate. Reduced chloride secretion at the apical surface of epithelial cells lining the secretory ducts of exocrine glands results in reduced hydration and fluidity of various secretions. However, in the sweat gland duct, CFTR is implicated in chloride absorption, an event important to spare salts during sweating. An abnormally high salt concentration in the sweat is still an obligate test to diagnose CF. Sticky, viscous and dehydrated secretions cause a plethora of tissue alterations including pancreas insufficiency and fibrosis, biliary cirrhosis, male infertility and obstructive intestinal diseases. In the lung, sticky secretions favor recurrent infections and an excessive inflammatory response resulting in lung insufficiency and fibrosis.
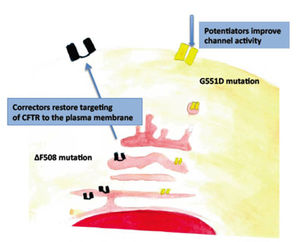
Recent progress in targeting and increasing the function of CFTR sheds new hopes for more rational therapies. Mutations in the ABCC7 gene may affect CFTR functions in different ways. The missense mutation G551D, that is present in 4-5 % of CF patients, results in the synthesis of a CFTR protein that reaches the plasma membrane, but displays a reduced channel activity (see figure). A drug targeting CFTR, VX-770 (Ivacaftor/Kalydeco) was reported to augment the chloride-transport activity of airway epithelial cells with the G551D-CFTR mutation in vitro, and to improve the lung function in CF patients carrying this mutation (1).Despite ABCC7 was discovered in 1989, no progress has been made in the gene therapy for this disease and treatments are still based on the use of antibiotics for lung infections, pancreatic supplements for malabsorption, chest physical therapy to favor expulsion of lung secretion and aerosolized DNase to severe sticky DNA present in the airway secretions.
Notably, treated patients showed a marked reduction (about 45 mmole/liter) in the sweat chloride concentration, a finding proofing that the drug targeted CFTR in vivo. Drugs increasing the chloride-transport activity of CFTR are called “potentiators” but can be used only in patients carrying ABCC7 mutation having effects similar to the G551D one. However, more than 50% of CF patients carry the ∆F508 mutation that is responsible for misfolding of CFTR and its retention in the endoplasmic reticulum (ER) (see figure). Thus, drugs to be used in these patients must be “correctors”, i.e. cause the correct targeting of the protein to the plasma membrane. To force a misfolded protein to leave the ER, go to the Golgi and then travel to the cells surface seems a very hard task. However a very promising compound, VX-809, showed to be able to correct the ∆F508 CFTR in vitro (2, 3) and in February 2013 Vertex Pharmaceuticals Inc, the VX-809 producer, began a phase III clinical trial in patients with two copies of the ∆F508 mutation. In the trial, VX-809 was used in combination with Ivacafactor because ∆F508 CFTR displays also reduced channel activity. If successful this trial will represent a breakthrough for the treatment of CF.
CFTR also transports bicarbonate. Recent evidence suggests that bicarbonate secretion is essential to disaggregate and reduce the density of intestinal CF mucus (4, 5). Additionally, in CF pigs the diminished pH of airway surface liquid reduces its antimicrobial capacity establishing a link between a defective CFTR, reduced bicarbonate secretion and lack of eradication of lung pathogens (6). How much and how many times per day bicarbonate can be aerosolized to the lung without adverse effects is absolutely unknown. The effects of bicarbonate on mucus viscosity and lung host defenses look however so strong to encourage studies along this line.
- Ramsey BW et al. (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. New Engl J Med 365:1663-1672.
- Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, Lukacs GL. (2013) Mechanism-based corrector combination restores ∆F508 -CFTR folding and function. Nat Chem Biol. 9: 444-454.
- He L, Kota P, Aleksandrov AA, Cui L, Jensen T, Dokholyan NV, Riordan JR (2013) Correctors of ∆F508 -CFTR restore global conformational maturation without thermally stabilizing the mutant protein. FASEB J. 27: 536-545.
- Garcia MA, Yang N, Quinton PM (2009) Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119:2613-2622.
- Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjövall H, Hansson GC (2013) Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209:1263-1272.
- Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487:109-115.