How do eukaryotic cells regulate the size and activity of different compartments to couple functional competence and homeostasis? How do they readapt to fulfil novels physiological tasks? Over the last years, some answers to these fundamental questions came from studies on the secretory system.
Let us start from the beginning, the endoplasmic reticulum (ER), where secreted proteins fold and assemble before being dispatched forward to the Golgi (if native) or otherwise back to the cytosol for degradation (1-2). To guarantee folding efficiency, chaperones and enzymes must outnumber cargo clients. A suitable balance is maintained by the unfolded protein response (UPR). This astonishing multidimensional pathway exploits virtually all available regulatory levels to maintain an efficient protein factory, acting on chromatin structure, transcription, translation and many posttranslational mechanisms. At least 3 sensors -Perk, Ire1 and ATF6- are on the look for aberrant proteins, or normal ones in excess (3).
When activated, they transiently inhibit translation, to relieve the pressure, while favouring the synthesis of folding assistants to fix unfolded molecules. If also increased degradation fails to solve the problem, apoptosis ensues to protect the organism against potentially obnoxious proteins. Branches of the UPR are utilized during development (3-4), a prototypic case being provided by the metamorphosis that B-lymphocytes undergo as they differentiate into plasma cells. The spectacular development of the secretory path finalized to massive Ig secretion requires Ire1 and XBP1. Interestingly, the Perk pathway is disposable, likely because attenuating translation is quite contradictory for plasma cells, each meant to release several thousands of antibodies per second (5).
Having invested in building a large ER, adequate efflux must be guaranteed. Therefore, downstream stations must coordinately develop. But how do cells sense and compensate the increased Golgi-incoming fluxes? Alberto Luini and co-workers discovered that KDEL receptors (KDELR) -besides retrieving chaperones to the ER- activate a Golgi pool of G proteins, accelerating Golgi traffic (6). This elegant mechanism couples production and delivery in the ER factory. There is clearly more to be learned. Can all KDEL-bearing proteins, or rather a few in particular, start the response? Is the latter linked to ER calcium storage-signaling? Is the redox gradient existing between cytosol and secretory pathway also involved?
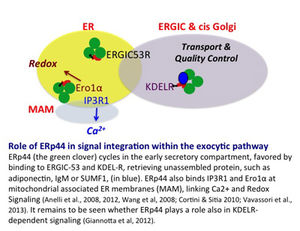
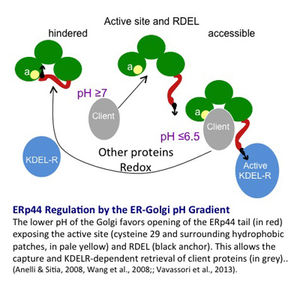
It is no coincidence that I mention calcium and redox signaling. We recently discovered an ESC-resident chaperone, ERp44, endowed with many properties indeed (see 7 and references therein). At the lower pH of the Golgi, ERp44 change conformation, simultaneously exposing its substrate binding site and RDEL localization motif. As such, ERp44 retrieves unpolymerized IgM or adiponectin subunits to the ER. At the neutral ER pH, clients are released to attempt polymerization, and ERp44 interacts with ERGIC53 to regain its patrolling position in the Golgi (8).
But that’s not all. ERp44 also binds and regulates IP3R1 and Ero1 oxidases in mitochondrial associated membranes, thus linking calcium and redox signaling/homeostasis (9). ERp44 thus emerges as a pivotal signal integrator relay in the early secretory compartment, shuttling through the cisGolgi, ER and MAMs. Does it also play a special role in KDELR signalling? Can it be a target in ER storage disorders?
References
- Sitia R and Braakman I. (2003). Quality control in the endoplasmic reticulum protein factory. Nature 426, 891-4.
- Brodsky JL. (2012) Cleaning up: ER-associated degradation to the rescue. Cell. 151:1163-7.
- Walter P, Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science. 334:1081-6
- Moore KA, Hollien J. (2012) The unfolded protein response in secretory cell function. Annu Rev Genet. 46:165-83.
- Masciarelli S, Fra AM, Pengo N, Bertolotti M, Cenci S, Fagioli C, Ron D, Hendershot L and Sitia R 2010. CHOP-independent apoptosis and pathway-selective induction of the UPR in developing plasma cells Molec. Immunol. 47:1356-65.
- Pulvirenti T, Giannotta M, Capestrano M, Capitani M, Pisanu A, Polishchuk RS, San Pietro E, Beznoussenko GV, Mironov AA, Turacchio G, Hsu VW, Sallese M, Luini A. (2008) A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat Cell Biol. 2008 Aug;10(8):912-22.
- Cortini M and Sitia R. (2010). From antibodies to adiponectin: role of ERp44 in sizing and timing protein secretion. Diab. Obes. And Metab. 12, 39-47
- Vavassori S, Cortini M. Masui S, Sannino S. Anelli T. Caserta I, Fagioli C, Mossuto MF, Fornili A. van Anken E, Degano M, Inaba K and Sitia R A pH-Regulated Quality Control Cycle for Surveillance of Secretory Protein Assembly. Molecular Cell In press
- Anelli T, Bergamelli L, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R and Sitia R (2012) Ero1a Regulates Ca2+ Fluxes at the Endoplasmic Reticulum-Mitochondria Interface (MAM). Antioxid. Redox Signal 16, 1007-87
- Giannotta M, Ruggiero C, Grossi M, Cancino J, Capitani M, Pulvirenti T, Consoli GM, Geraci C, Fanelli F, Luini A, Sallese M. (2012) The KDEL receptor couples to Gαq/11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 31:2869-81